As the push for greater reproducibility, competition for grants, and pressure to publish increase, automation to achieve greater standardization in Western Blot processing is now a necessity and not a luxury.
Years ago, bench scientists and grad students, myself included, still made acrylamide gels themselves. We spent countless of hours in the darkroom developing films. In those days, we used the Western blot primarily for qualitative purposes.
During the incubation part, we used to fiddle with Saran wrap. Then, we’d throw the membrane into the first cover from the pipet box lying around. It was the norm.
Most of the antibodies I was using worked well enough at room temperature. So, if a washing step was skipped or extended here there, or I used an antiquated rocker platform, it didn’t seem to affect results much. And those that didn’t work, we simply cast aside.
Fast forward to the pressures of today’s new norm in automated Western Blot processing.
As someone who has not even thought of Western blot for almost a decade, I was shocked to find out how much has changed.
Digital imagers have replaced film and densitometry to quantify the bands.
Fluorescence is gradually displacing chemiluminescence to detect the protein because the signal intensity is more linear.
Precast acrylamide gels make pouring the gels the old-fashioned way the relic of the past.
And, high-speed, high-quality, semi-dry, transfer apparatus eliminate the need to carry tanks full of transfer buffer back and forth.
All of these innovations are being fully embraced by the scientific community to address the reproducibility crisis.
Why?
Because the Western Blot procedure has been one of the most frequent culprits of the crisis!
The recent focus of the scientific community on the irreproducibility crisis has increased the appreciation of how minute details affect the outcomes of the Western Blot process.
Some of them wouldn’t have even occurred to me during my graduate years.
For example, the way we ensure even antibody distribution across the membrane matters. We know shaking the membrane side-to-side is better than rocking.
We know incubation temperatures also matter. Often, a temperature change to just 4 degrees increases the sensitivity of the blot signal because it’s less likely to be washed off at colder temperature.
Finally, we know that the shaking speed when we wash the membranes is critical. The shaking needs to be vigorous enough to wash off the non-specific signal.
Unfortunately, this is often hard to achieve using the conventional rockers without loss of liquid or the membrane being tossed out of the box.
Compared to other parts of the protocol, decanting and filling the trays with different solutions and then just letting the trays rock seems deceptively straightforward.
Yet this part of the Western Blot process involves “babysitting” the blot.
Sometimes, it requires changing the solutions every 5-10 minutes.
In addition, timing the steps accurately distracts researchers from doing other experiments or reading and writing the papers.
And, let’s face it: In reality, very few people are willing to go back in forth to a cold room every 5-10 minutes during washes.
We have designed BlotCycler™ with these challenges in mind.
Our mission was to give more love to this simple, powerful, technique in life science research that (undeservedly) got such a bad rap recently.
Each of these variables has been automated by the BlotCycler ™
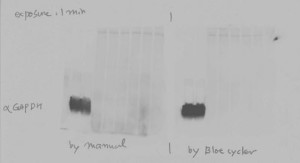
Automated Western Blot Processing with the BlotCycler enables researcher to produce accurate, reliable results
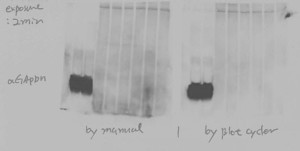
Consistent, reliable, reproducible results with the BlotCycler Automated Western Blot Processing
We’ve automated the most tedious part of the Western blot process for you. And we’ve tested it. Repeatedly.
What we found is that automated Western Blot processing using the BlotCycler™ increases reliability, productivity, and reproducibility.
The BlotCycler™: the new standard in Western Blot research.



