A discussion of research comparing Western blot automation to the manual approach.
This research was previously reported on the Rockland Immunochemicals website
Immunodetection of proteins on the Western blot is often performed manually and involves multiple steps that easily introduce bias and errors. The multi-step traditional manual Western blotting technique is both an effort and time consuming process. One of the problems of manual processing is the variability of results between blots.
In this experiment, we compared the traditional manual Western blotting and a novel type of automatic Western blotting by using BlotCycler™ processor developed by Precision Biosystems, and DyLight™ fluorochrome conjugated secondary antibodies developed by Rockland Immunochemicals.
1. Testing Incubation with Different Blocking Buffers
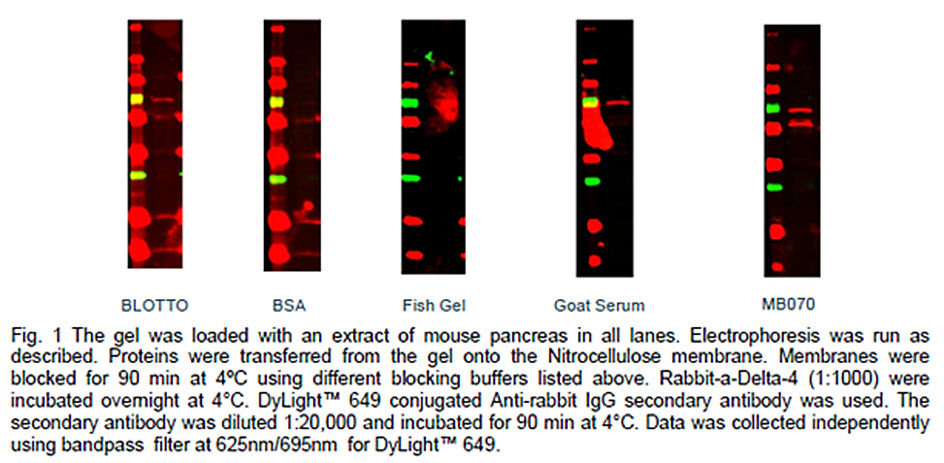
In order to obtain high quality results using the Western blot, it is important to select an efficient blocking buffer. A good blocking buffer maximizes the signal-to-noise ratio and won’t react with the targeted protein or primary or secondary antibodies. The efficiency of blocking depends on incubation conditions. We compared the efficiency of five commonly used blocking buffers to prevent nonspecific binding of the florescently labeled secondary antibody during automated processing.
2. Comparison of Western Blot Automation and Manual Blot Processing
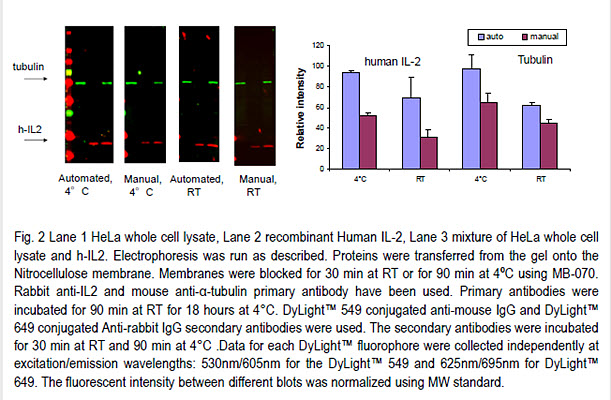
It is a common practice to incubate primary antibody at 4ºC and perform all other steps at RT.
However, this eliminates advantages of incubation at low temperature on background and signal intensity.
In preliminary experiments we noticed that performing all steps at 4ºC using the BlotCycler™ yields higher signal intensity and lower background than manual processing with primary antibody incubation at 4ºC and all other steps at RT.
Therefore we evaluated the role of automation in increased sensitivity and performed all steps both manual and automated processing entirely at 4ºC or RT.
3. Reproducibility of Automated Blot Processing
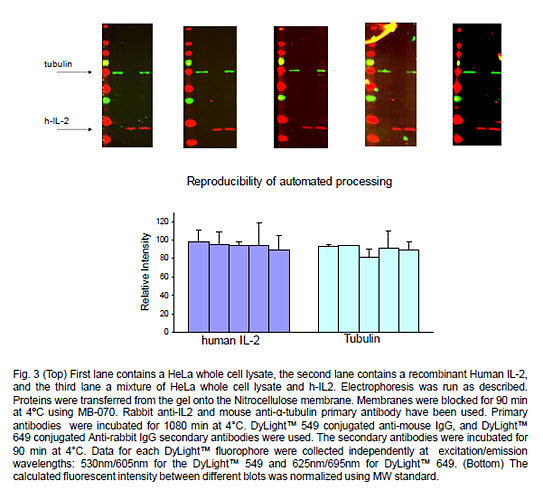
One of the problems of manual processing is the variability of results between blots. In this experiment we processed several identical blots using the BlotCycler™
In addition to MW standard, each blot contained three lanes:
- HeLa whole cell lysate
- Recombinant protein Human IL-2
- A mixture of HeLa whole cell lysate and h-IL-2.
The five identical blots were incubated with a mix of rabbit anti-IL-2 and mouse and anti-Α-tubulin and then probed with corresponding secondary antibodies: DyLight™ 549 conjugated anti-mouse IgG and DyLight™ 649 conjugated anti-rabbit IgG.
The automated processing showed excellent reproducibility as a result of precise timing and consistency of solution changes, efficient washing, and elimination of operator error ensuring standardization of the results.
4. Optimization of the Antibody Concentration
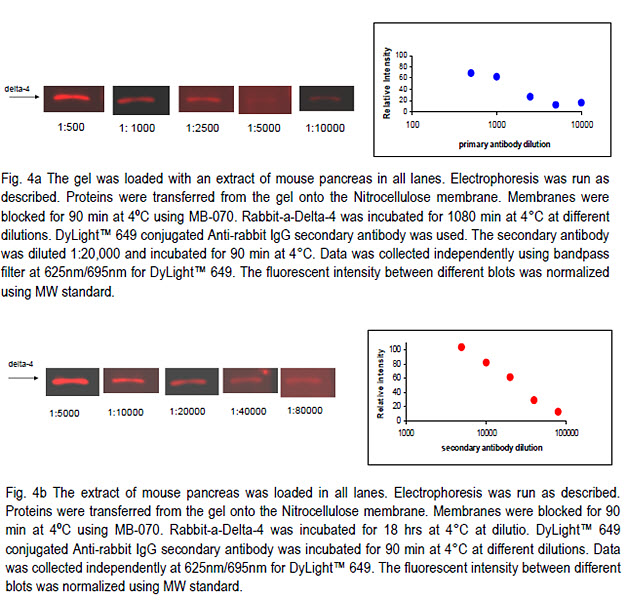
In this experiment we prepared ten identical strips each containing two lanes: MW markers and extract of mouse pancreas.
The blots were probed with rabbit andit-Delta4 primary antibody and DyLight™649 conjugated anti-rabbit secondary antibody.
In the first experiment we used five different dilutions of primary antibody (Fig. 4a) and a single dilution of secondary antibody (1:20,000).
In the second experiment we selected a single dilution of primary antibody (1:1000) and six different dilutions of the secondary antibody (Fig. 4B)
Using the BlotCycler™ we were able to construct the titration curve for both primary and secondary antibodies that can be used to select optimal antibody concentration depending on the experiment objective.
Optimal primary and secondary antibody concentrations depend on the antibody’s specific activity.
Optimization is essential when one or more of the experimental variables such as the antigen, the primary or the secondary antibody is changed. However, optimization of antibody concentration can be tedious and difficult to compare when multiple blots are processed in slightly different conditions.
The BlotCycler™ allows simultaneous processing up to six different blots with six different secondary or primary antibodies at the same time with high reproducibility.
Summary
We used fluorescent labeled secondary antibodies to compare manual and automated processing of the Western Blot.
The multi-color visualization presented here demonstrates that the BlotCycler™ is able to produce clear and high quality results.
This allows straight forward process for identification of individual proteins.
Using two identical protocols, we showed that the BlotCycler™ increases the signal to noise ratios by reducing background and increasing signal intensity.
The BlotCycler™ provides extremely good reproducibility that is probably related to minimal variability in washing and incubation times.
The BlotCycler™ can be used to optimize the concentrations of primary and secondary antibodies. This has the potential to provide a huge cost saving by decreasing the amount of antibody used.
Our results indicate that manual and automatic processing were comparable at RT.
However, automatic processing yields consistently higher signal at 4ºC.
Western blot automation increases the signal and improves the quality of the final Western blot at 4ºC.
Western blot automation using BlotCycler™ showed excellent reproducibility and can be efficiently used for optimization of primary and secondary antibodies concentration.
The BlotCycler™ offers a time saving alternative to manual processing of fluorescent Western blot by providing very low background staining, high signal-to-noise ratio, an ability to multiplex detection and excellent reproducibility.