Detection of low rEPO concentrations in samples can only be successful when immunoblots show an excellent signal-to-noise ratio that are reliably reproduced. In this research, an evaluation of the current published procedures was undertaken to determine possible ways to optimize the procedures.
By using the BlotCycler for incubation and washing, these researchers were able to increase reliability and reproducibility of their results while economizing the labor time used to produce results.
Incubation steps:
The blotted membrane was incubated in 50mL of blocking solution on an orbital shaker for 1 hour at RT.
Then, the membrane was rinsed briefly with PBS and incubated in reducing buffer (10 mM DTT in PBS) for 15 minutes at 37°.
Subsequent incubation and washing steps were performed in the BlotCycler™ automated Western blot processor. at 4° C.
Incubation times for primary and secondary antibodies were adjusted to ensure optimal signal-to-noise ratio by using the BlotCycler™!
After reducing, the membrane was briefly washed three times with PBS and placed into a PBS filled tray in the BlotCycler™.
25 mL of primary antibody (1:1000 in PBS plus 1% LFM) and secondary antibody solution (1:100 000 in PBS plus 1% LFM) were filled into the designated tubes of the BlotCycler™.
PBS was filled to the was buffer tank.
Using the BlotCycler for incubation, it was programmed as follows:
The membrane was incubated in PBS for 5 minutes followed by 3 hours of incubation with the primary antibody. The secondary antibody was incubated for 13.5 hours.
Both antibody incubations were followed by nine, 5 minute washing steps.
Detection:
After washing, the membrane was ready for the detection procedure.
The membrane was removed from the BlotCycler™ and excess PBS was drained.
Then the membrane was placed on a glass plate, and three mL of detection solution was applied evenly across the membrane.
Results and discussion:
The original protocol stipulated a microfiltration step before ultrafiltraion to avoid clogging of the ultrafiltration unit.
These investigators were able to omit this step by centrifuging the samples for 12 minutes at 4000g.
This modified sample preparation procedure reduced both cost and labor, while signal intensities of EPO were not impaired in downstream analysis.
Gel electrophoresis of large sample numbers can be analyzed best with Midi-gels because Midi-gels can fit 16 samples together with standards and a negative quality control. (Figs. 1A and 1B)
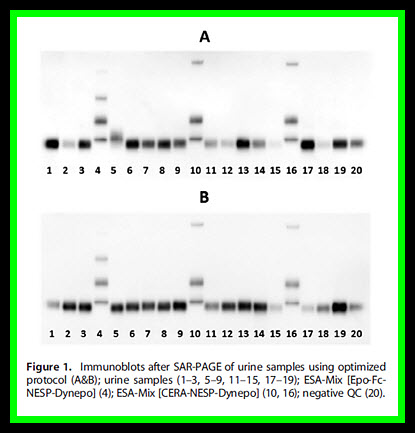
Image courtesy of wileyonlinelibrary.com/journal/dta
Drug Testing and Analysis 2015,7, 1014-1016
In routine analysis, 4 Midi-gels (64 samples) per day were easily handled by a single operator. The blot was conducted as a single blot.
After blotting the use of the BlotCycler for incubation and washing steps increased the reproducibility of results significantly. When analyzing four gels at once, the BlotCycler™ economized labor time drastically.
The advantage of using this new optimized approach? A team of three operators could perform 1000 EPO screening analyses from urine per month!
Because the sample preparation procedure was revised and simplified, the researchers saved both labor and material costs. Additionally, washing and incubation steps of the immunoblots carried out by the BlotCycler™ automated Western blot processor optimized the workflow, reproducibility, and quality of the results.
What’s in your lab?
Ready to start automating your Western blot process?
Contact us at 1-888-490-4443




