Since 1975 when Edward Southern introduced his powerful DNA transfer and probing technique, Western blots have become a workhorse in bioscience research.
The elegance of the technique is truly awe-inspiring. Proteins are separated electrophoretically on acrylamide gel. Then, they’re transferred to a nitrocellulose or PVDF membrane by capillary action in the same way that blotting paper absorbs ink. Afterward, the blotted membrane can be incubated with a specific antibodies. It all sounds so simple.
So, what’s the big deal about Western Blot Minimum Reporting Standards?
The problem is and has been, controlling for the many variables that affect the outcome of the blot.
Recently, there have been so many research retractions involving Western blots, the technique is described as one of the most untrustworthy research methods.
These authors suggest that Western blots have become a joke in published papers due to the failure of researchers to perform proper controls, or replicates. The problem is compounded by the failure of reviewers to demand Western Blot Minimum Reporting Standards data. And to be sure, retracted research gives rise to doubt in the scientific community and cries of outrage from the public. The state of the science leaves many bench scientists wondering if there’s a witch hunt on its way. And it’s no wonder! Being able to trust the experimental data is critical for experimental research.
However, as Kenneth Pimple reminds us
“Distinguishing poorly designed research from faked data is hard, but it is generally more difficult to prove misconduct than to identify the cause of irreproducibility. . . . Irreproducibility and data fabrication/falsification have the same result: Inaccurate publications. In general, research that cannot be reproduced should be put on the side or in the trash, but when there is probable cause that research misconduct is involved, it should be investigated.”
Research antibodies are a major contributor to the current reproducibility crisis. A wide variety of issues can jeopardize antibody-dependent experiments—including age, stability, inappropriate storage and handling, and lot-to-lot variation—resulting in widespread lack of trust in their quality control and specificity.
To that end, in September of this year, an ad hoc International Working Group for Antibody Validation met for the first time.
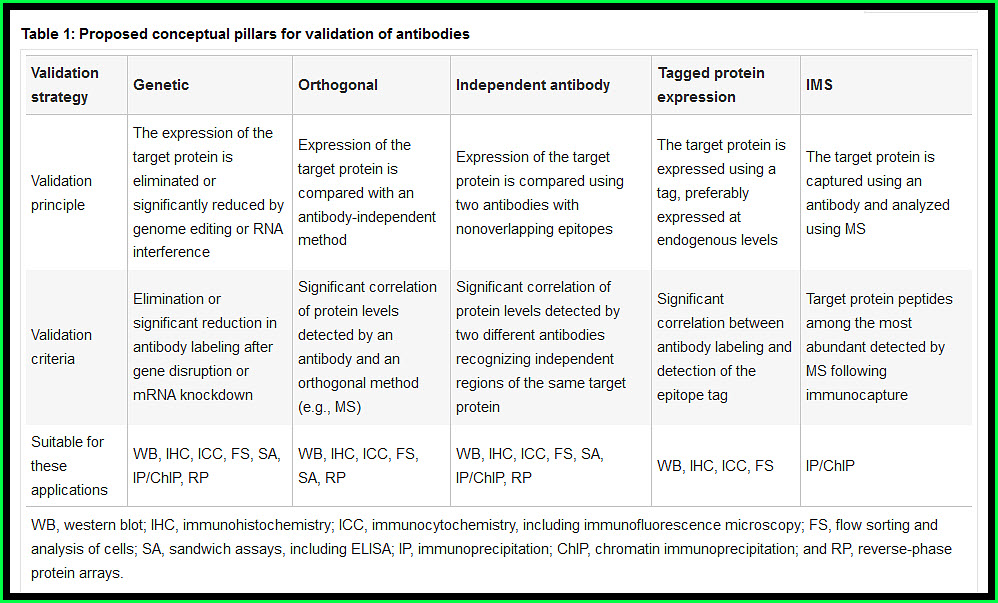
Their purpose: to formulate the best approaches for validating antibodies used in common research applications and to provide guidelines that ensure antibody reproducibility. They recommend five conceptual ‘pillars’ for antibody validation used in an application-specific manner.
In this report, they described a series of scientifically sound approaches for antibody validation. While each of these approaches provides sufficient evidence for validation in a particular application, the authors suggest that combinations of multiple validation strategies may increase the confidence in antibody specificity.
But even the use of properly validated antibodies in a non-validated context causes problems.
Although the suggested Western Blot Minimum Reporting Standards documentation will allow other researchers to better reproduce and confirm or fail to confirm previously published data, limitations due to experimental error cannot be accounted for. The use of different experimental conditions adds to potential variability and affects the linearity and sensitivity of the Western blot. Simple technical factors often confound the electrophoretic resolution or antibody detectability of the researchers’ protein solutions. Sometimes it’s from unsuitable sample concentrations. At other times, it’s buffer incompatibility or simple processing errors. Sometimes it’s the absence of calibration markers or treatment controls.
So, until the adoption of uniform Western Blot Minimum Reporting Standards, there’s a risk that Western blot irreproducibility will continue to be the norm.
And yet, trying to keep track of all the data in one place can be a bit of a challenge.
Therefore, based on the suggestions of Gilda, et al., we’ve created this handy Western Blot Minimum Reporting Standards Worksheet PDF. We created this specifically for use with the BlotCycler™ automated Western blot processor that allows to eliminate simple processing errors and reduce variability due to stringent control of washing conditions. But, it’s also handy for folks who may be doing manual processing as well.