A recent article published in the journal Drug Testing and Analysis
(Volume 8, Issue 11-12, November-December 2016, Pages 1131–1137) “Evaluation of AMGEN clone 9G8A anti-Epo antibody for application in doping control“ demonstrates how the BlotCycler™ is currently being used in one area of research.
In a previous post, we discussed some of the recent advances in anti-doping research: Improved detection of EPO in blood and urine based on novel Velum SAR precast horizontal gels optimized for routine analysis. Blood doping is the misuse of certain techniques and/or substances to increase red blood cell mass. For athletes, this allows the body to transport more oxygen to muscles and therefore increase stamina and endurance.
There are three widely known substances or methods used for blood doping, namely, erythropoietin (EPO), synthetic oxygen carriers, and blood transfusions. Each of these substances is prohibited under WADA’s List of Prohibited Substances and Methods.The report of this current research builds on that previously published work.
The aim of the current project was to compare the sensitivity and specificity of clone 9G8A anti-EPO antibody with a commercially available clone AE7A5 as an alternative antibody for anti-doping control purposes.
The antibody was developed and characterized by the Amgen company and is mentioned in TD2014EPO as possible alternative to AE7A5, a currently utilized antibody against EPO. Although AE7A5 exhibits high sensitivity, its cross-reactivity with several E. coli and S. cerevisiae proteins is a major drawback. Contrary to AE7A5 that was developed against EPO’s N-terminus, 9G8A was derived using the full-length recombinant human EPO. It is directed against a linear epitope containing amino acids E13, L16, and L17.
Tris (PlusOne) and the BlotCycler™ were obtained from GE Healthcare (Uppsala, Sweden) and Precision Biosystems (Mansfield, MA), respectively.
For single-blots, the PBS-washed membranes were transferred into the trays of the BlotCycler™ and were automatically processed with primary antibody in 1% LFM/PBS (1 µg/mL for clones AE5A7 and 9G8A; 300 min; coldroom) and HRP-labelled secondary antibody (1:100.000 dilution of a stock solution containing 0.8 mg/mL; 690 min; coldroom), with PBS-washing steps between the incubation steps (9x5min each).
Sensitivity comparison of clones AE7A5 and 9G8A anti-EPO antibodies on IEF-PAGE (Western double-blot. At a dilution of 1 µg/mL the sensitivity of clone 9G8A (B) was about 45% of AE7A5 (A). Tested was a mixture of BRP-EPO and NESP ranging from 100 pg (lane 1) to 25 pg (lane 2) and 6.25 pg (lane 3). Shown is an exposure time of 30 seconds.
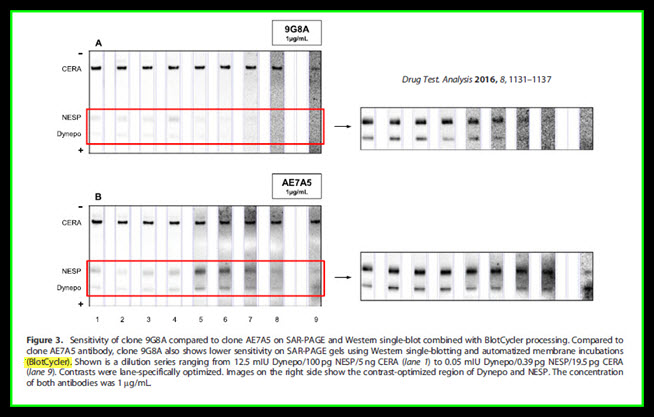
The sensitivity of 9G8A was further evaluated by performing SAR-PAGE and a recently published single-blotting protocol, which applies an instrument for automatizing incubation in primary and HRP-labelled secondary antibodies and all washing steps (BlotCycler™).
Again, 9G8A showed considerably lower sensitivity than AE7A5 as shown in Figure 3.
Sensitivity of clone 9G8A compared to clone AE7A5 on SAR-PAGE and Western single-blot combined with BlotCycler™ processing. Compared to clone AE7A5 antibody, clone 9G8A also shows lower sensitivity on SAR-PAGE gels using Western single-blotting and automatized membrane incubations (BlotCycler™). Shown is a dilution series ranging from 12.5 mIU Dynepo/100 pg NESP/5 ng CERA (lane 1) to 0.05 mIU Dynepo/0.39 pg NESP/19.5 pg CERA (lane 9). Contrasts were lane-specifically optimized. Images on the right side show the contrast-optimized region of Dynepo and NESP. The concentration of both antibodies was 1 µg/mL.
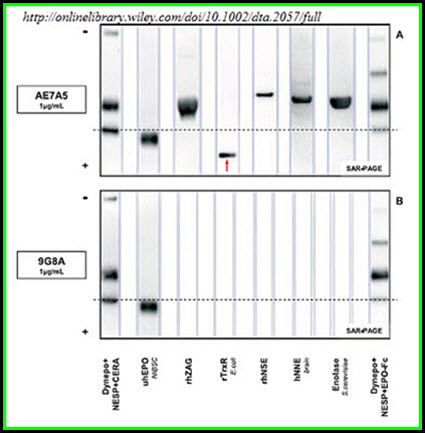
Figure 5
Figure 5 discussion
Non-specific binding behaviour of clones AE7A5 anti-EPO antibody on SAR-PAGE with single-blot and BlotCycler membrane processing. A. Clone AE7A5 showed strong non-nspecific binding to E. coli rTrxR and rhNSE. Weaker non-specific binding was detectable for rhZAG, hNNE and S. cerevisiae enolase. B. In contrast, no non-specific binding was detected for clone 9G8A. The images show 12.5mIU Dynepo/58.5 pg NESP/78 pg CERA (lane 1), 5mIU NIBSC (lane 2), 5 µg rhZAG (lane 3), 0.1 µg E. coli rTrxR (lane 4), 0.1 µg of rhNSE (lane 5), 15 µg of hNNE (lane 6), 5 µg of S. cerevisiae enolase (lane 7), and 10.3mIU Dynepo/50.3 pg NESP/82.5 pg EPO-Fc (lane 8). The exposure time was 15 sec for AE7A5 and 60 sec for 9G8A. Lanes were individually contrast optimized with GASepo software in order to reveal weak signals. The arrow marks the position of rTrxR on SAR-PAGE.
Conclusion
Five test proteins (hZAG, E. coli TrxR, hNSE, hNNE, S. cerevisiae enolase), which showed medium to strong non-specific interactions with clone AE7A5 anti-EPO antibody, were evaluated regarding their binding behavior to the non-commercial anti-EPO antibody clone 9G8A. Contrary to AE7A5, clone 9G8A was developed using full length EPO with the correct N-terminal sequence. The antibody showed neither on SDS-, SAR- or IEF-PAGE cross-reactions with the five proteins. However, the sensitivity was lower compared to clone AE7A5. By applying immunoaffinity purification before electrophoretic separation, the non-specifically bound proteins were removed and no longer interfered with correct identification of endogenous and recombinant erythropoietins by AE7A5. This purification step has already been mandatory for all molecular mass based separations used for the detection of rEPO misuse in the past.
When these researchers evaluated the potential use of clone 9G8A for doping control purposes, they used the BlotCycler™ automated Western blot processor to enhance the reliability and validity of their findings.
What’s in your lab?
Ready to get started automating your Western blot process?
Contact us at 1-888-490-4443