When researchers investigating Rapamycin treatment of healthy pigs subjected to acute myocardial ischemia-reperfusion, they used the BlotCycler™ Automated Western Blot Processor for increased reliability and validity.
Research Application Note: Rapamycin Treatment of Healthy Pigs Subjected to Acute Myocardial Ischemia-Reperfusion Injury Attenuates Cardiac Functions and Increases Myocardial Necrosis
Published in final edited form as: Annals of Thoracic Surgery. 2014 Mar; 97(3): 901–907.
Antonio D Lassaletta, MD, Nassrene Y Elmadhun, MD, Arthus V D Zanetti, Jun Feng, MD, PhD, Javier Anduaga, B.S., Reginald Y. Gohh, MD, Frank W Sellke, MD, and Cesario Bianchi, MD, PhD
Molecular Studies
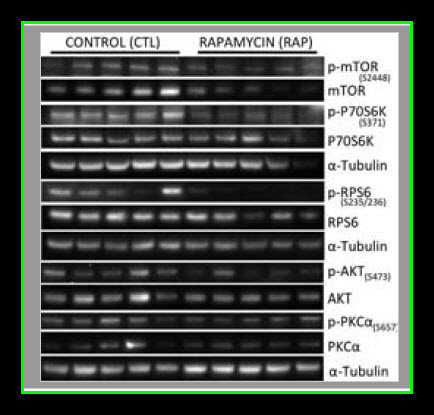
Myocardial lysates were prepared in Radio-Immunoprecipitation Assay (RIPA) buffer (Boston BioProducts) as previously described12. Sixty micrograms of RIPA soluble myocardial lysates from a remote left ventricular (RLV) area were fractionated by SDS-PAGE using a 4–12% Bis-Tris gel (Invitrogen) and transferred to PVDF membranes (Millipore, Bedford, MA). Using an automated western blot processor (Precision Biosystems, Mansfield, MA), membranes were incubated with antibodies against mTOR, p-mTOR, p-S6, P70S6K, p-70S6K, LC3A, LC3B, α-tubulin (all from Cell Signaling, Danvers, MA), and S6 (Santa Cruz Biotechnology, Santa Cruz, CA) at dilutions recommended by the manufacturer, followed by the appropriate horse radish peroxidase-linked secondary antibodies (Jackson ImmunoResearch, West Grove, PA), visualized via enhanced chemiluminescense (ECL) and recorded with a digital imaging system (G-Box, Syngene, Cambridge, England). Raw data were collected as arbitrary light units, averaged and quantified microdensitometrically using ImageJ 1.40g (National Institutes of Health, Bethesda, MD).
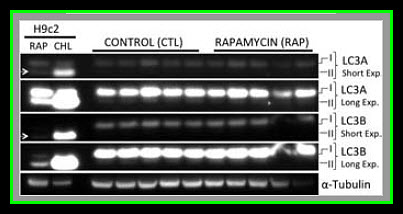
Photo courtesy of
Annals of Thoracic Surgery and can be found at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3943541/
Comment
Our results suggest that pre-treatment with rapamycin prior to acute myocardial IRI is detrimental to both cardiac function as well as myocardial survival in healthy pigs. It remains to be determined if an in vivo autophagic flux was induced by rapamycin treatment, but there was no evidence of autophagosome accumulation as LC3AII and LC3BII levels were similar between controls and the rapamycin treated group. It worthwhile to clarify that higher levels of LC3A/B II does not necessarily reflect an increase in autophagy since autophagic activity is also dependent on autophagosome-lysosome fusion (autolysosome formation) that can be inhibited under some conditions such as with the administration of chloroquine (Appendix Figures 1–3) and a high fat diet. In addition, chloroquine treated cells had higher levels of LC3A/B II (autophagosome accumulation, Appendix Figures 1–3) but decreased autophagic activity due to inhibition of lysosomal activity, lysosomal autophagosome fusion, and less autolysosome formation. The attenuated hemodynamics and microvascular function observed in the current study are likely the effects of rapamycin resulting from mTOR inhibition, regardless of whether or not autophagic flux was altered.
It is prudent to discuss the potential clinical implications of these preliminary findings as rapamycin is commonly used at doses comparable to those in the current study for both induction and maintenance immunosuppression after solid organ transplantation. Our findings do suggest that patients taking rapamycin may be at risk for worse outcomes, specifically in regard to infarct size and hemodynamic function after an acute cardiac event. This is especially concerning if one considers the increased risk of such events with patient taking rapamycin.



